Clinical Trial
About the clinical trial
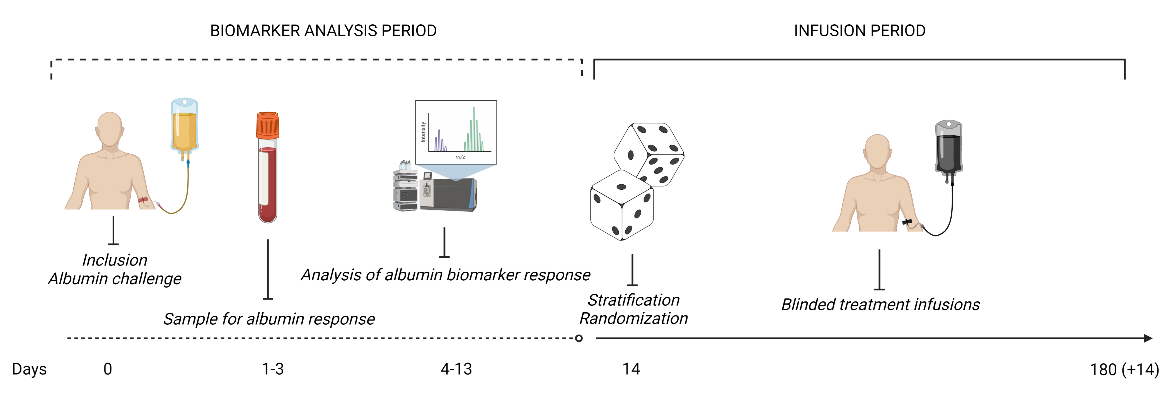
The aim of the Alb-trial within the MICROB-PREDICT project is to validate a biological marker to predict how well the body responds to treatment with albumin in patients with cirrhosis. The biological marker has been developed within the MICROB-PREDICT project and will be tested in a European randomized, double-blinded, placebo-controlled trial with patients enrolled from twelve European centers.
In this trial, the effect of long-term administration of human albumin will be investigated in a cohort of decompensated cirrhotic patients stratified by their expected treatment response to human albumin. The aim is to identify patients who are likely to clinically benefit from the long-term treatment with human albumin, resulting in reduced or improved symptoms and lower risk of death. The results of this trial are expected to be a major step forward towards personalized treatment for these patients.
Study sites
The study is performed in out-patient clinics at twelve clinical study sites across seven European countries and has started on 27/02/2024.
Denmark
Odense University Hospital, Odense
Prof. dr. Aleksander Krag
Herlev Hospital, Herlev
Dr. Mette Lehmann Andersen
Germany
Universitätsklinikum Münster
Prof. dr. Jonel Trebicka
Charité Hospital, Berlin
Prof. dr. Cornelius Engelmann
University Hospital Jena
Prof. dr. Alexander Zipprich
Belgium
University Hospital Leuven
Prof. dr. Wim Laleman
Hungary
Debreceni Egyetem, Debrecen
Prof. dr. Maria Papp
The Netherlands
Leiden University Medical Center, Leiden
Dr. Minneke Coenraad
Alrijne Hospital, Leiderdorp
Dr. Sunje Abraham
United Kingdom
King’s College London
Prof. dr. Debbie Shawcross
Spain
Fundacio Privada Clinic per a la Recerca Biomedica, Barcelona
Prof. dr. Pere Ginés
Hospital del Mar, Barcelona
Dr. Montserrat Garcia Retortillo